
NUCLEAR FUSION is the process by which two light nuclei combine
to form a heavier nucleus, releasing a large quantity of energy.
![]()
In the bland lexicon beloved of physicists, a plasma is defined as an "ionized gas." The idea is to distinguish it from the ordinary or "neutral" gases of various kinds that we breathe, burn in stoves and torches, and use to puff up lighter-than-air balloons. In a neutral gas, each atom has the same number of negatively charged electrons orbiting its nucleus as positively charged protons in that nucleus. While such a gas may possess plenty of chemical activity along with dynamical effects like fluid turbulence, it shows little response to electric and magnetic fields and is practically unable to conduct electricity.
The gas jumps to life once enough of its atoms become ionized by losing one or more of their electrons. The ionization can take place either because the gas is very hot (so that the atoms smash together, jarring loose electrons as if they were rugby players' teeth), very rarefied (so that as electrons are occasionally liberated by any process, they virtually never encounter an ion with which to recombine) or subjected to some outside source of energy (such as electric fields so strong they rip electrons from atoms or photons of light that kick the electrons out of their orbits). The resulting plasma consists of interpenetrating, and interacting, clouds of freely roaming positive and negative charges whose motions both generate electromagnetic fields and are, in turn, influenced by them.
A gas's properties can shift from neutral to plasma with lightning quickness. The cliché is meant literally. The churning winds in thunderclouds, like rabbit's fur rubbed on a glass rod, can charge up parts of the clouds either positively or negatively with respect to the ground. When enough charge builds up somewhere, it can drive a weak, branching electrical discharge that grows invisibly toward the ground. As soon as one of the branches touches a tree or a church steeple or an unfortunate golfer on a fairway, the excess charge blasts through the electrical connection that has been opened, ionizing and heating the atmospheric gases along its path. The terrific heat creates a shock wave that plows into the surrounding neutral gas and generates the sound that we call thunder. If the golfer hears it, then she has gotten off lucky in her quick introduction to plasma physics.
In checking for the presence of a plasma under more leisurely circumstances, there actually exists a test more rigorous than simply requiring that an appreciable fraction of the background gas is ionized. The test has to do with the tendency of a cloud of charged particles to gang up and "shield," or form an
electrostatic sheath around, any particular charged object that is placed in the cloud-say, a metallic probe or a specific ion singled out from all the rest. In a vacuum or a normal gas, the strength of the electric field around such an ion falls off approximately as the square of the distance from the ion.
But in a plasma, the field is almost completely shielded beyond a distance
called the Debye length. In fact, it is only when the Debye shielding length
is smaller than the cloud of charged particles-smaller, for instance, than
the laboratory chamber in which they're produced-that physicists strictly
refer to the collective ensemble as a plasma. (By this criterion, a plasma
can coexist with a background of ordinary gas, flowing through it and interacting
with it only via collisions.) In technical detail, the more charged particles
per unit volume are present to neutralize electric fields, the shorter the
Debye length; and the higher a plasma's temperature, allowing its particles
to resist the temptation to congregate around a bare charge, the longer
the Debye length. The key, however, is the notion that particles in a plasma
act in concert, behaving as a collective beast that seems to have a mind
of its own.
![]()
As one manifestation of such behavior, a skin or sheath forms around a plasma when it is in contact with a solid surface. Plasma sheaths, like so many collective effects in plasma, can be either a blessing or a curse to the engineers who try to put this elusive state of matter to work for them. When a plasma is created above a
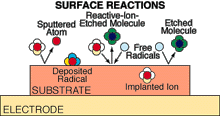
PLASMA DISCHARGES used in materials processing cause bombardment
of solid surfaces with free radicals and ions, inducing a variety of processes
such as ion implantation, sputtering, etching and deposition.
semiconducting wafer in a microprocessing fab, the strong electric fields in the sheath at the wafer's surface accelerate plasma ions nearly straight downward, and some of them zip through the gaps in a "photoresist," or mask, that bears the pattern of an integrated circuit. Those ions crash into the silicon-based material and pump energy into the chemical reactions that etch the narrow, deep trenches-often less than a micron across-out of which the circuits are made. Earlier techniques, based on wet chemistry, tended to undercut the trench walls and couldn't pack as much memory and logic circuitry onto a wafer.
Related methods are supplementing and supplanting wet chemistry in applying thin, high-quality coatings to everything from jet engine components to human prosthetics. Unfortunately, the physics of a plasma's edge doesn't always spin a happy tale for the engineers, and such boundary effects