SM J04 2
From QED
Consider a gas of N nonrelativistic fermions with spin 1/2 and mass m initially at zero temperature and confined in a volume V0 and kept at.
a. Express the kinetic energy of the gas in terms of N and V0.
b. What is the pressure of the gas? You can assume here that the gas is ideal.
c. Now the gas is allowed to expand to the volume 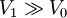 without any energy exchange with the outside world.
Calculate the temperature of the gas after it will
reach an equilibrium due to weak interactions between
the fermions.
without any energy exchange with the outside world.
Calculate the temperature of the gas after it will
reach an equilibrium due to weak interactions between
the fermions.
d. What is the pressure of the gas in the final state.
Define 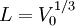 as the length of one side of the cube
containing the gas. We can write the wavefunction of
one of the fermions as:
as the length of one side of the cube
containing the gas. We can write the wavefunction of
one of the fermions as:
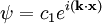
Because we are limited to volume V0 , we must have:
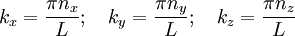
So that the total energy is:
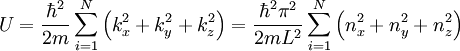
Since T=0, the fermions are in their minimum occupancy, but since they have spin 1/2 each level can be filled by only two fermions. Thus:
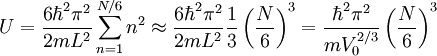
Where we used the limit of very large N for the second equality.
We can use the ideal gas equation:
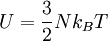
along with:
to find:
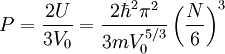
Since there can be no energy exchange, and the number is fixed, we still have:
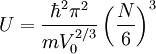
So that:
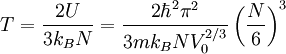
Again we just use the ideal gas equation:
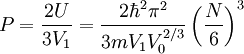
This page was recovered in October 2009 from the Plasmagicians page on Prelim_J04_SMT2 dated 02:18, 13 August 2006.












